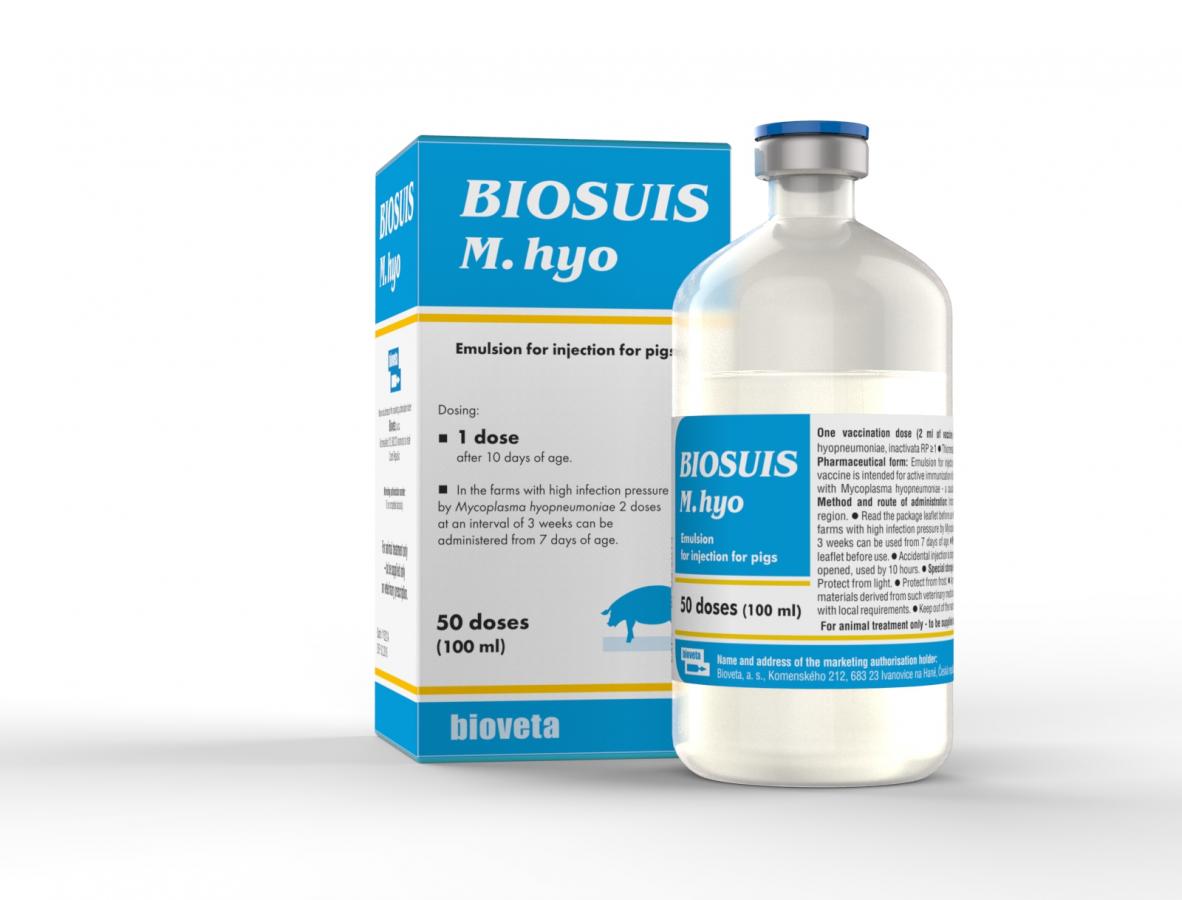
BIOSUIS M. hyo emulsion for injection for pigs
Homepage Products Veterinary products BIOSUIS M. hyo emulsion for injection for pigs
Inactivated vaccine against Enzootic Pneumonia of Pigs (EPP).
| type of preparative: | Vaccines |
|---|---|
| target species animals: | Pig |
Pharmaceutical form
Emulsion for injection.
Appearance: Milky liquid of yellowish-white to light pink colour without sediment or with small amount of sediment dispersing after shaking.
Composition
Inactivated Mycoplasma hyopneumoniae RP ≥ 1*
*RP = Relative potency (ELISA test) as compared with the reference serum obtained after vaccination of mice with a vaccine batch that has successfully passed the challenge test on the target species.
Indication
For active immunization of fattening pigs to mitigate the effects of infection with Mycoplasma hyopneumoniae - a causative agent of Enzootic Pneumonia of Pigs.
The onset of immunity has been demonstrated in vaccinated piglets 14 days after vaccination or revaccination if 2 doses were applied.
Vaccine ensures duration of immunity during fattening period.
Dosage, application and vaccination scheme
Intramuscularly, preferably into the paraauricular region, vaccination dose: 2 ml.
Vaccination:
1 dose should be administered to piglets from 10 days of age.
In the farms with high infection pressure by Mycoplasma hyopneumoniae 2 doses at an interval of 3 weeks can be administered from 7 days of age.
Selection of the vaccination scheme depends on knowing the disease incidence on a particular farm.
Storage
Store in a refrigerator (2 °C - 8 °C).
Protect from light.
Protect from frost.
Withdrawal period
Zero days.
Shelf life
Shelf-life of the veterinary medicinal product as packaged for sale: 2 years.
Shelf life after first opening the immediate packaging: 10 hours.
Package
10 ml, 100 ml, 250 ml.
Not all pack sizes may be marketed.